The formation of structures that have a controlled helicity continues to be a challenging area in the field of polymer and supramolecular chemistry. We report a series of helical copolyaniline nanofibers of formed by copolymerization of aniline with m-toluidine or o-toluidine, respectively. The helicity of the PANI nanofibers was induced by a chiral D-CSA dopant in the polymerization process, but could be inverted by copolymerization with m-toluidine. Copolymerization with o-toluidine resulted in a helicity similar to that of PANI. Theoretical simulations revealed that the steric hindrance of the methyl group at the meta- position of the phenyl ring played a dominant role in the helical inversion. It is of great importance that upon simply introducing a methyl substituent at the meta- position of the phenyl ring, helical inversions are observed not only for the copolymer conformation but also for their aggregated nanofibers, which provides a subtle method of tuning the induced supramolecular chirality.
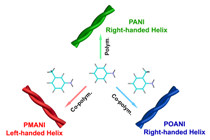 Schematic representation of supramolecular chirality tuning by methyl substitution.
http://www3.interscience.wiley.com/cgi-bin/fulltext/121682562/PDFSTART
Schematic representation of supramolecular chirality tuning by methyl substitution.
http://www3.interscience.wiley.com/cgi-bin/fulltext/121682562/PDFSTART
The formation of structures that have a controlled helicity continues to be a challenging area in the field of polymer and supramolecular chemistry. We report a series of helical copolyaniline nanofibers of formed by copolymerization of aniline with m-toluidine or o-toluidine, respectively. The helicity of the PANI nanofibers was induced by a chiral D-CSA dopant in the polymerization process, but could be inverted by copolymerization with m-toluidine. Copolymerization with o-toluidine resulted in a helicity similar to that of PANI. Theoretical simulations revealed that the steric hindrance of the methyl group at the meta- position of the phenyl ring played a dominant role in the helical inversion. It is of great importance that upon simply introducing a methyl substituent at the meta- position of the phenyl ring, helical inversions are observed not only for the copolymer conformation but also for their aggregated nanofibers, which provides a subtle method of tuning the induced supramolecular chirality.

Schematic representation of supramolecular chirality tuning by methyl substitution.
http://www3.interscience.wiley.com/cgi-bin/fulltext/121682562/PDFSTART
 Close Page
Close Page- Text Size: A A A
 Printer Friendly
Printer Friendly
