Alzheimer’s disease (AD) is a neurodegenerative disease related to beta-amyloid peptide (Aβ), and its high morbidity and long duration make it a severe burden on economics, society and family. Beta amyloid peptides and other amyloidogenic proteins could form diversity of aggregate morphologies, such as nanofibrils, spherical aggregates, annular protofibirils, and plaques, which are all reported to be correlated to neurotoxicity at different level. The neuron death will finally lead to senile dementia. It is vital for early stage diagnosis and treatment of amyloidosis to fully understand the mechanism of the aggregation process and also put forward an effective way to modulate the peptide aggregation behaviors.
Great progress has been made recently on the mechanism and the modulation of the peptide aggregations by molecular chaperon effect. Researchers from National Center for Nanoscience and Technology have reported their contributions on the assembly mechanism of A42 related to AD (J. Mol. Biol. 2009, 388, 894) and amylin related to type II diabetes (J. Struct. Biol., 2009, 167, 209) by using scanning tunneling microscopy. These progresses provided new venue for the structural analysis and the interpretation of the assembly mechanism of the peptide assemblies with beta-sheet secondary structures. Furthermore, they reported a new method for effective modulation of aggregation of amyloid proteins by chaperon-like molecules. The assembly characteristics can be tuned from molecular level to two-dimensional assembly level and finally to three-dimensional aggregate level.
The modulator effect of peptide assemblies are demonstrated both for the surface assembling processes and aggregation in solution. Such observations on the modulator effect could provide effective approach toward modulating amyloid peptide aggregation via diversity of interactions such as hydrogen bond, hydrophobic interaction, etc. It could be also beneficial for control and prevention, potential drug designs and treatment relating to neurodegenerative diseases. The current research achievement has been published on Nano Letters (Nano Lett. 2009, 9(12), 4066-4072).
The research works mentioned above are financially supported by National Basic Research Program of China from Ministry of Science and Technology, Chinese Academy of Sciences, and National Natural Science Foundation of China.

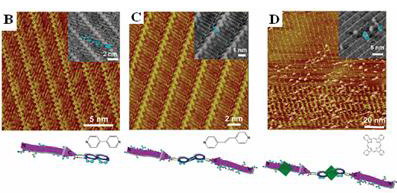 Scanning tunneling microscopic (STM) images of modulation of peptide assemblies by molecular modulators.
Scanning tunneling microscopic (STM) images of modulation of peptide assemblies by molecular modulators.
(A) Assembly of A peptide. (B, C and D) Modulation of peptide assemblies by different molecular modulators, 4,4-bipyridyl (DP), 1,2-di(4pyridyl)ethylene (DPE) and phthalocyanine (Pc), respectively.
Contributors:
CAS Key Laboratory for Biological Effects of Nanomaterials & Nanosafety
CAS Key Laboratory for Nanocharacterization
Great progress has been made recently on the mechanism and the modulation of the peptide aggregations by molecular chaperon effect. Researchers from National Center for Nanoscience and Technology have reported their contributions on the assembly mechanism of A42 related to AD (J. Mol. Biol. 2009, 388, 894) and amylin related to type II diabetes (J. Struct. Biol., 2009, 167, 209) by using scanning tunneling microscopy. These progresses provided new venue for the structural analysis and the interpretation of the assembly mechanism of the peptide assemblies with beta-sheet secondary structures. Furthermore, they reported a new method for effective modulation of aggregation of amyloid proteins by chaperon-like molecules. The assembly characteristics can be tuned from molecular level to two-dimensional assembly level and finally to three-dimensional aggregate level.
The modulator effect of peptide assemblies are demonstrated both for the surface assembling processes and aggregation in solution. Such observations on the modulator effect could provide effective approach toward modulating amyloid peptide aggregation via diversity of interactions such as hydrogen bond, hydrophobic interaction, etc. It could be also beneficial for control and prevention, potential drug designs and treatment relating to neurodegenerative diseases. The current research achievement has been published on Nano Letters (Nano Lett. 2009, 9(12), 4066-4072).
The research works mentioned above are financially supported by National Basic Research Program of China from Ministry of Science and Technology, Chinese Academy of Sciences, and National Natural Science Foundation of China.


(A) Assembly of A peptide. (B, C and D) Modulation of peptide assemblies by different molecular modulators, 4,4-bipyridyl (DP), 1,2-di(4pyridyl)ethylene (DPE) and phthalocyanine (Pc), respectively.
CAS Key Laboratory for Biological Effects of Nanomaterials & Nanosafety
CAS Key Laboratory for Nanocharacterization
 Close Page
Close Page- Text Size: A A A
 Printer Friendly
Printer Friendly
