Jin-Song Hu Ling-Ling Ren An-Min Cao Li-Jun Wan* and Chun-Li Bai*
Environmental problems associated with organic pollutants and toxic water pollutants provide the impetus for sustained fundamental and applied research in the area of environmental remediation. Semiconductor photocatalysis offers the potential for complete elimination of toxic chemicals due to its efficiency and potentially broad applicability. Therefore it is very essential to develop a cost-effective mass-production method of semiconductor photocatalysts with highly catalytic activity and easy separation.
Now a simple mass-production and low-cost self-assembly synthesis of ZnS nanoporous nanoparticles (NPNPs) composed of hexagonal ZnS nanocrystals with 3?C5 nm size has been developed by Prof. Bai and Prof. Wan in Institute of Chemistry CAS. The uniform spherical NPNPs are monodisperse and possess surface areas on the order of 156 m2g-1 (Fig. 1). The advantages of the ZnS NPNPs as semiconductor photocatalysts are (1) High surface-to-volume ratios and effectively preventing the nanoparticles from aggregation so as to result in high catalytic activity; (2) The profitability of size-quantized nanometer-sized semiconductor particles involving the higher redox potentials due to the increase in band-gap energy (the energy difference between LUMO and HOMO) which in turn improves charge transfer rates in the system and the drastically reduction of volume recombination (radiationless recombination of the electron?Chole pair within the semiconductor particle); (3) Easier recycle and separation than common NCs due to the large diameter of NPNPs; (4) Good dispersity and proper dimension without the requirement of constant stirring or dark reaction for adsorption of substrates. As a result the ZnS NPNPs show higher photocatalytic activity than Degussa P25 titania or ZnS nanocrystals in the photodegradation of eosin B at ambient temperature (Fig. 2).
ZnS photocatalysts have been used not only for photodegradation of organic dyes also for photoreductive H2 production and photoreduction of CO2 phototransformation of various organic substrates photoreductive dehalogenation of halogenated benzene derivatives photocatalytic removal of water pollutants and photocatalytic reduction of toxic metal ions and so on. Therefore the ZnS NPNPs are expected to find many interesting applications in semiconductor photocatalysis and environmental remediation as well as nanodevices as a result of its high surface-to-volume ratios monodispersion rich photocatalytic and luminescent properties.
Angew. Chem. Int. Ed. 2005 44(8) 1269-1273
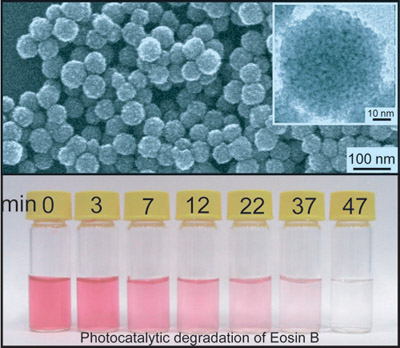
Fig. 1 SEM and TEM image of ZnS NPNPs and the optical photo of photocatalytic degradation of Eosin
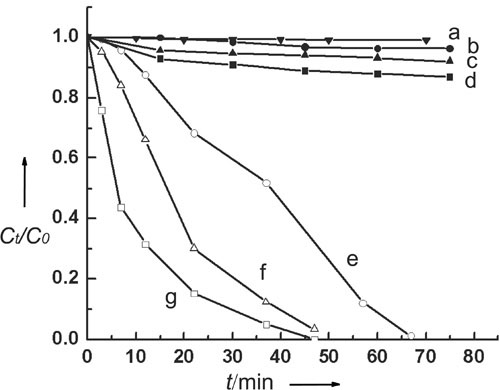
Fig. 2 The curves of photodegradation of Eosin with e) Degussa P25 titania f) ZnS NCs and g) ZnS NPNPs.
Jin-Song Hu Ling-Ling Ren An-Min Cao Li-Jun Wan* and Chun-Li Bai*
Environmental problems associated with organic pollutants and toxic water pollutants provide the impetus for sustained fundamental and applied research in the area of environmental remediation. Semiconductor photocatalysis offers the potential for complete elimination of toxic chemicals due to its efficiency and potentially broad applicability. Therefore it is very essential to develop a cost-effective mass-production method of semiconductor photocatalysts with highly catalytic activity and easy separation.
Now a simple mass-production and low-cost self-assembly synthesis of ZnS nanoporous nanoparticles (NPNPs) composed of hexagonal ZnS nanocrystals with 3?C5 nm size has been developed by Prof. Bai and Prof. Wan in Institute of Chemistry CAS. The uniform spherical NPNPs are monodisperse and possess surface areas on the order of 156 m2g-1 (Fig. 1). The advantages of the ZnS NPNPs as semiconductor photocatalysts are (1) High surface-to-volume ratios and effectively preventing the nanoparticles from aggregation so as to result in high catalytic activity; (2) The profitability of size-quantized nanometer-sized semiconductor particles involving the higher redox potentials due to the increase in band-gap energy (the energy difference between LUMO and HOMO) which in turn improves charge transfer rates in the system and the drastically reduction of volume recombination (radiationless recombination of the electron?Chole pair within the semiconductor particle); (3) Easier recycle and separation than common NCs due to the large diameter of NPNPs; (4) Good dispersity and proper dimension without the requirement of constant stirring or dark reaction for adsorption of substrates. As a result the ZnS NPNPs show higher photocatalytic activity than Degussa P25 titania or ZnS nanocrystals in the photodegradation of eosin B at ambient temperature (Fig. 2).
ZnS photocatalysts have been used not only for photodegradation of organic dyes also for photoreductive H2 production and photoreduction of CO2 phototransformation of various organic substrates photoreductive dehalogenation of halogenated benzene derivatives photocatalytic removal of water pollutants and photocatalytic reduction of toxic metal ions and so on. Therefore the ZnS NPNPs are expected to find many interesting applications in semiconductor photocatalysis and environmental remediation as well as nanodevices as a result of its high surface-to-volume ratios monodispersion rich photocatalytic and luminescent properties.
Angew. Chem. Int. Ed. 2005 44(8) 1269-1273
Fig. 1 SEM and TEM image of ZnS NPNPs and the optical photo of photocatalytic degradation of Eosin
Fig. 2 The curves of photodegradation of Eosin with e) Degussa P25 titania f) ZnS NCs and g) ZnS NPNPs.
 Close Page
Close Page- Text Size: A A A
 Printer Friendly
Printer Friendly
