Immune checkpoint blockade (ICB) is one of the primary methods of tumor immunotherapy. However, clinical data have shown that only a portion of patients respond to ICB treatment. Since the tumor's immune-suppressive environment plays a crucial role in ICB therapy, finding ways to effectively reshape this environment and increase the response rate to ICB treatment has become a key focus.
In a study published in Nature Nanotechnology, a research team led by Prof. WANG Hai and NIE Guangjun from the National Center for Nanoscience and Technology of the Chinese Academy of Sciences, collaborating with Prof. RAN Haitao from Chongqing Medical University, developed three types of nanostructures that combine L-phenylalanine with metal ions. This innovative design can effectively reshape the tumor's immune-suppressive environment and significantly enhance the effectiveness of ICB immunotherapy.
Dendritic cells (DCs) play a crucial role in the immune response against cancer and infections. Mature DCs are key to activating tumor-specific immunity. Over the past decade, two main activation modes have been discovered to induce DC maturation and trigger the initial immune response: pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).
Besides these danger signals, many functions of DCs, including maturation, cytokine production, and migration, are also regulated by electrical signals in the body. The concentration of potassium and calcium ions in DCs is closely related to their maturation process. However, the movement of metal ions in and out of cells is strictly controlled by ion channels, and there is currently no effective way to activate these channels.
To activate the potassium ion channels on the DC membrane, the researchers used magnesium ions, iron ions, and zinc ions to coordinate with L-phenylalanine, creating three types of nanostructures: nanospheres (Ph-Mg), nanoneedles (Ph-Fe), and nanosheets (Ph-Zn). They found that these nanomaterials could enter cells through pinocytosis and caveolae-mediated endocytosis, but they were unstable in acidic environments.
Computer simulations indicated that the disassembled nanostructures would release as dimers of metal ion-chelated L-phenylalanine. These dimers can bind to the S4 transmembrane region of the potassium ion channel (Kv1.3), causing the Kv1.3 structure to change, widening the channel and activating the potassium ion channel. The outflow of potassium ions, along with the influx of calcium ions triggered by depolarization, activates the NF-κB signaling pathway regulated by calmodulin, promoting DC maturation and triggering the secretion of pro-inflammatory cytokines.
In addition, the researchers found that the uptake of nanostructures by DCs may induce the release of cathepsin B, which, along with the outflow of potassium ions, activates the inflammasome pathway closely related to DC maturation. They showed that nutrient restriction could enhance the uptake of nanomaterials by DCs, upregulate calmodulin expression, and degrade IkBα, further strengthening the NF-κB pathway.
"Currently, most protein regulation focuses on developing inhibitors. Finding ways to activate protein functions is still challenging," said Prof. WANG, the lead author of this study. "Fortunately, we discovered that using nanomedicine can precisely activate the Kv1.3 potassium ion channel in DCs. This activation reverses the tumor's immune-suppressive environment and enhances the effectiveness of immune checkpoint inhibitors."
This study develops metal ion-amino acid nanostructures that can regulate ion channel structures, promoting the activation of the innate immune response by adjusting the potassium and calcium ions in DCs. This reshapes the tumor's immune-suppressive environment, providing a new strategy for improving the effectiveness of ICB therapy.
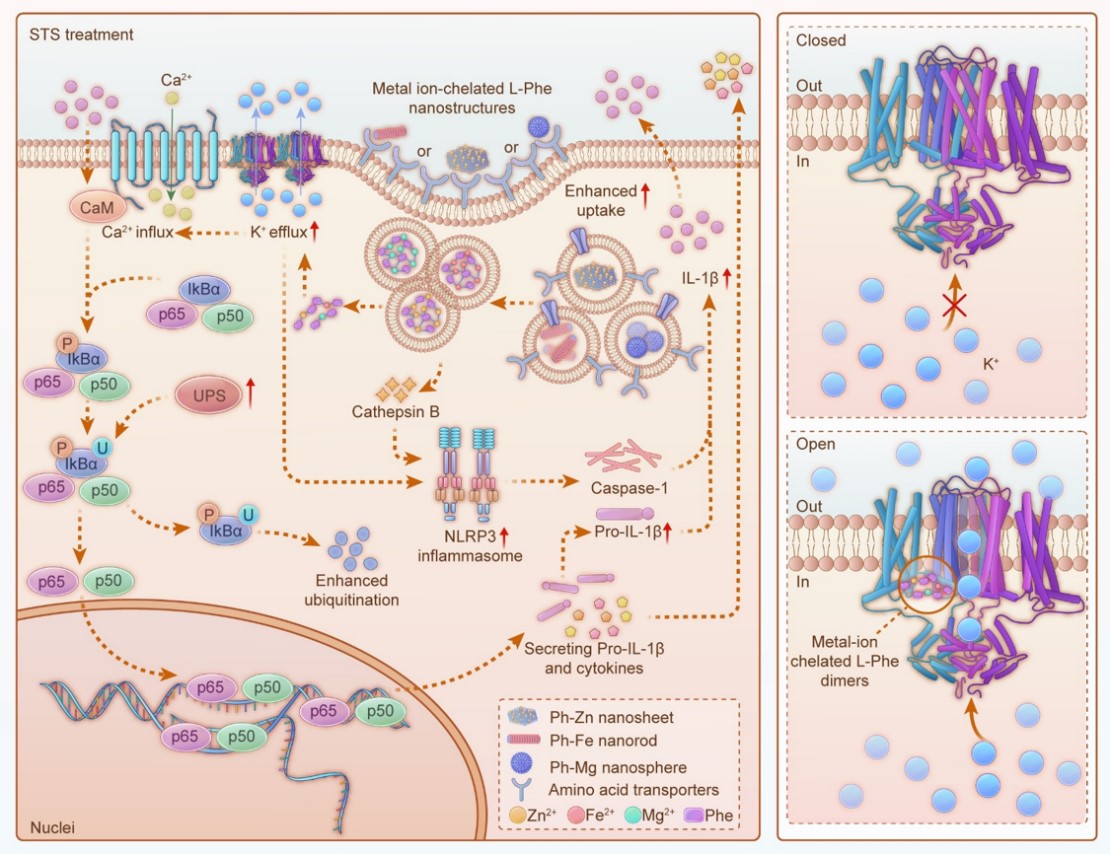
Schematic Illustration of Enhanced Tumor Immunotherapy Using DC Cell Therapy Mediated by Metal Ion-Chelated L-Phenylalanine Nanocomplexes (Image by WANG Hai et al)
Contact: WANG Hai
National Center for Nanoscience and Technology (NCNST)
E-mail: wanghai@nanoctr.cn




