In a step forward for improving cancer immunotherapy outcomes, researchers at the National Center for Nanoscience and Technology (NCNST) have now developed a biomimetic physical barrier (BPB) that temporarily blocks T cell-tumor cell interactions, effectively delaying T cell exhaustion and enabling a stronger, more sustained immune response. The study was led by Prof. LIANG Xingjie team and recently published in the Proceedings of the National Academy of Sciencesof the United States of America.
While cancer immunotherapies like immune checkpoint blockade and chimeric antigen receptor T cell therapy have reshaped modern oncology, their efficacy in solid tumors remains limited. One major reason is that T cells rapidly become “exhausted” after infiltrating the tumor tissue, losing their ability to kill cancer cells. This exhaustion is driven in part by persistent interactions between T cells and tumor cells, which involve multiple immunosuppressive signaling pathways that apply brakes to the immune response.
The team of Prof. LIANG Xingjie at NCNST, together with Prof. GONG Ningqiang at the University of Science and Technology of China, drew inspiration from the fibrotic barriers in solid tumors, which physically hinder interactions between immune cells and tumor cells, contributing to immune evasion. “What if we could harness the concept of physical blocking to modulate the immune microenvironment?” said Prof. LIANG. “What if a controllable barrier could regulate the T cell-tumor cell interaction and give T cells a break to delay their exhaustion?”
To test this idea, the team designed a thermoresponsive hydrogel-based BPB that mimics the fibrotic matrix. After being injected into the tumor, the BPB undergoes a sol-to-gel transition at body temperature, forming a temporary “protective zone” that physically blocks T cells-tumor cell interactions. This delays T cell exhaustion process and allows them to accumulate in a more functional state. When sufficient T cells were gathered, a mild dose of near-infrared light is applied to trigger the gel-to-sol transition of BPB, removing the barrier and re-exposing the T cells to tumor cells. At this point, the accumulated T cells exhibit enhanced cytotoxic activity and improved antitumor efficacy in multiple tumor models.
The team uncovered the underlying mechanism of therapeutic effect of BPB. During the BPB construction, more stem-like progenitor exhausted T (Tpex) cells accumulated in the tumor tissue. Once the BPB was removed, these Tpex cells induced a stronger and more sustained immune response against the tumor tissue. “We were excited to uncover this mechanism, which fundamentally explains how the BPB works,” says Dr. ZHANG Yuxuan, the first author of the study.
The results suggest that temporarily blocking T cell-tumor cell interactions can reprogram the immune response toward a more durable and effective state. “A brief blockade, not to compromise, but a setup for a stronger comeback,” said Prof. LIANG. “BPB strategy gives the immune system a chance to gather force and preserve strength.”
A conceptual framework is described by researchers for their BPB strategy. “We call this strategy ‘immunological rhythm control’. By modulating the interaction between T cells and tumor cells, we intervene in the process of T cell exhaustion and preserve T cell functional activity to achieve a more effective immune response,” explains Prof. GONG.
Controlled modulation of the T cell-tumor cell interaction represents a promising step toward sustainable cancer immunotherapy. The BPB strategy may also be combined with a variety of other immunotherapeutic approaches in the future to enhance treatment efficacy.
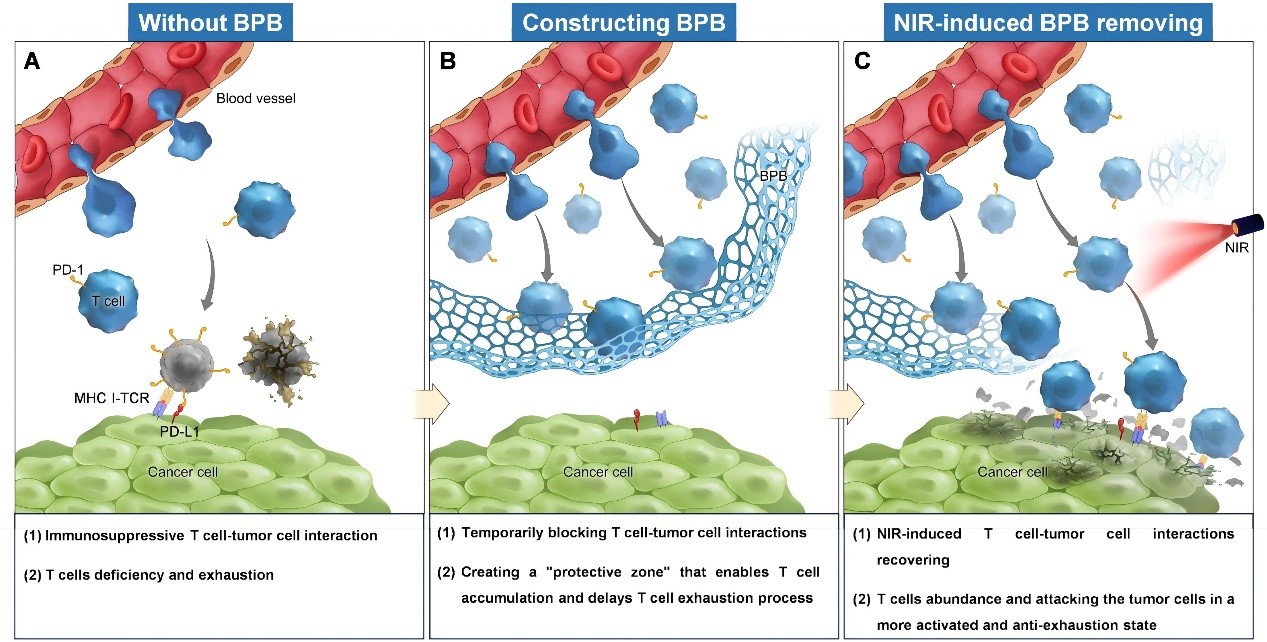
Schematic diagram showing the T cell fate with and without biomimetic physical barrier treatment (Image by LIANG Xingjie et al)
Contact: LIANG Xingjie
National Center for Nanoscience and Technology (NCNST)
E-mail: liangxj@nanoctr.cn




