Sepsis-induced immune dysfunction leaves patients vulnerable to life-threatening secondary infections. Although antibiotics remain the first-line clinical option, recent studies reveal that long-term immunosuppression during sepsis–marked by impaired macrophage antibacterial function–directly correlates with unfavorable patient outcomes, indicating that the antibiotic regimen falls short in preventing secondary infections. Although some macrophage-based therapies have been proposed to address sepsis-associated secondary infection, achieving a harmonious balance between infection control and alleviation of immunosuppression remains challenging.
In response to this problem, Prof. LIANG Xing-Jie's team from the National Center for Nanoscience and Technology, China, collaborating with Prof. LUO Yang from the Chongqing University, has developed a self-assembling peptide nanoparticle named "BATMAN" (bacteria-targeted transformable macrophage nanorejuvenator), to tackle sepsis-associated secondary infection by coordinating the arrest of invasive bacteria and rejuvenation of dysfunctional macrophages and compromised immune functions in vivo. The study was published in Science Translational Medicine.
"As a pivotal link between innate and adaptive immunity, in situ reprogramming of macrophages' antibacterial activity not only promotes bacterial clearance but also holds promise for rejuvenating the suppressed immune responses, thereby enhancing the host antibacterial defense. This is crucial in addressing secondary infections in sepsis." said Prof. LIANG. The BATMAN is designed to autonomously assemble through transformable peptide, comprising the bacteria-targeted ubiquicidin 29–41 (UBI29–41), assembly–driving β amyloid–derived peptide (FFVLK), bacterial lipase–sensitive cholesteryl hemisuccinate (Chems), and immunoglobulin G-derived natural phagocytosis-stimulating peptide (tuftsin).
The adept bacterial binding performance and bacterial lipase responsiveness support the deformation of BATMAN, contributing to the inside-out transformation and assembly to expose and cluster the concealed tuftsin peptides on bacterial surface for interaction with macrophage Fcγ receptors (FcγR). The tuftsin clusters resulting from this reorganization subsequently interact with the macrophage FcγR to improve the immune function, promote bacterial clearance, and thus alleviate sepsis-induced immunosuppression. In preclinical sepsis models afflicted with polymicrobial- and even multidrug-resistant pathogen-induced pulmonary infection, BATMAN treatment improved survival compared with antibiotic therapy and promoted restoration of antimicrobial defenses.
The findings highlight the importance of restoring compromised immune function in tackling sepsis-associated secondary infection. By integrating immune function modulation with bacterial invasiveness inhibition, BATMAN-based macrophage reprogramming strengthens the host's ability to eliminate bacteria and provide reliable protection against potential recurrent infections. This macrophage-targeted therapy represents a promising clinical strategy for secondary infections in patients with sepsis.
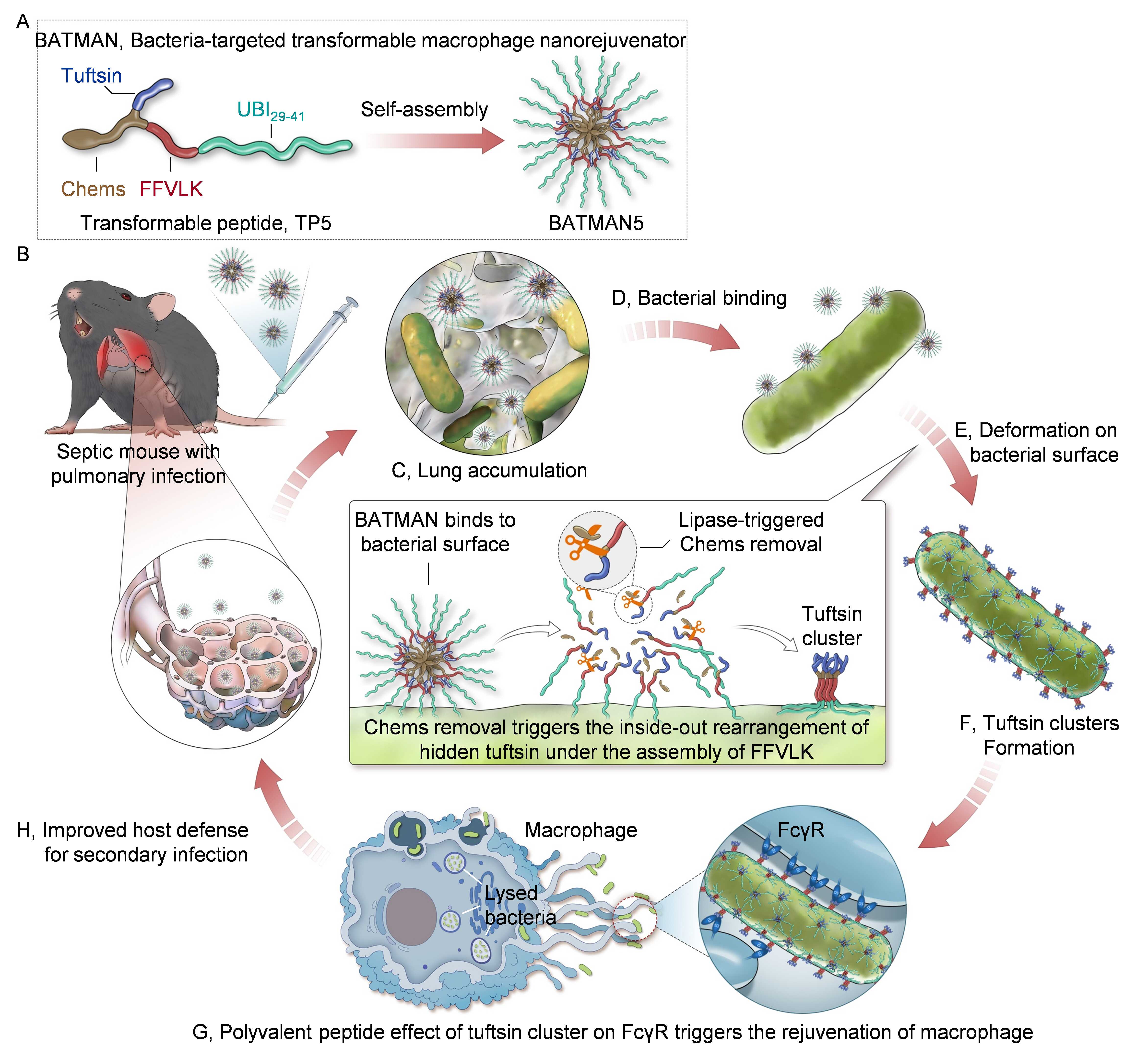
Schematic diagram showing the rejuvenation of dysfunctional macrophage by transformable BATMAN against sepsis-associated secondary infection (Image by LIANG Xing-Jie et al)
LIANG Xing-Jie
National Center for Nanoscience and Technology, China (NCNST)
E-mail: liangxj@nanoctr.cn




