Oxaliplatin (OXA) is a widely used third-generation platinum-based chemotherapeutic agent and a first-line treatment for colorectal cancer. However, its clinical application is severely limited by a major side effect: peripheral neuropathy. Up to 95% of patients undergoing OXA treatment experience cold allodynia—an extreme sensitivity to cold temperatures that triggers pain. This condition often leads to dose reduction or treatment discontinuation, compromising therapeutic outcomes. While previous research focused primarily on direct neuronal damage, the precise mechanisms driving this hypersensitivity have remained elusive.
Recently, a research team led by Prof. JI Tianjiao and Prof. NIE Guangjun from the National Center for Nanoscience and Technology, China (NCNST), Chinese Academy of Sciences (CAS), in collaboration with Prof. CHEN Hanqing from Capital Medical University and Dr. MIAO Jiamin from Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, made a significant breakthrough. Their study, published in Cell Biomaterials, reveals that dermal fibroblasts—cells in the skin's connective tissue—play a critical role in mediating OXA-induced cold allodynia.
The researchers discovered that free OXA tends to accumulate in the skin, specifically within dermal fibroblasts. This accumulation activates the NF-κB signaling pathway, which in turn upregulates the expression of the chemokine CCL2. The elevated CCL2 then interacts with CCR2 receptors on nearby nerve endings, activating the TRPM8 ion channel (a cold sensor) and triggering the sensation of pain. This establishes the "NF-κB-CCL2/CCR2/TRPM8" axis as a key molecular mechanism underlying the pathology.
To address this, the team utilized nanocarriers to reshape the drug's biodistribution. They demonstrated that nanoformulated OXA significantly reduces platinum accumulation in the dermis compared to the free drug. By preventing the buildup of platinum in fibroblasts, the nanoformulation effectively cuts off the pain-inducing signaling cascade at its source. Importantly, this reduction in side effects does not come at the cost of efficacy; the nanoformulated drug maintained potent antitumor activity in colorectal cancer models.
This study not only uncovers a novel peripheral mechanism for chemotherapy-induced neuropathy but also highlights the potential of nanomedicine to decouple toxicity from therapeutic efficacy. By optimizing drug delivery to avoid non-target tissues like the skin, this strategy offers a viable path toward safer and more tolerable cancer treatments.
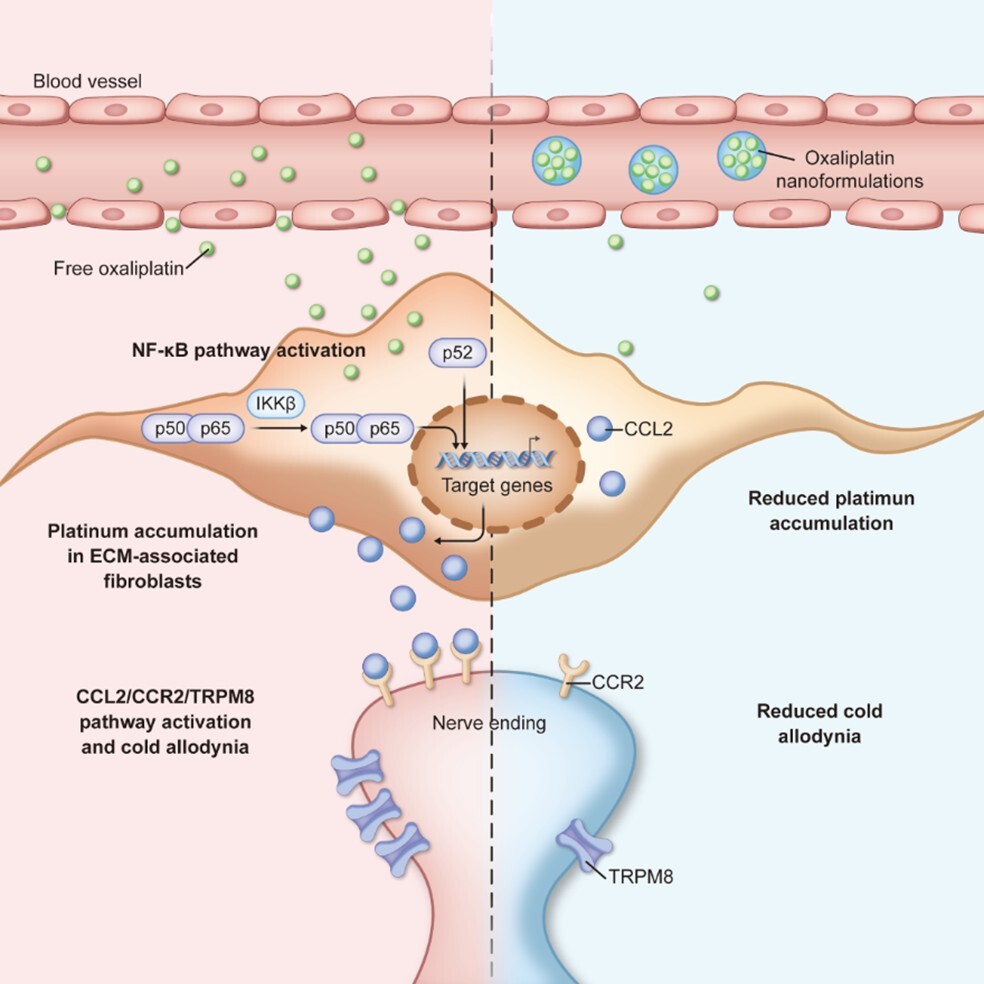
Figure. Nanoformulated oxaliplatin reduces platinum accumulation in dermal fibroblasts, inhibiting the NF-κB-CCL2 signaling pathway and alleviating cold allodynia. (Image by ZHANG Shuhui et al.)
Contact: JI Tianjiao
National Center for Nanoscience and Technology (NCNST)
E-mail: jitj@nanoctr.cn




