A research team led by Prof. CHEN Chunying from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences (CAS) reported the mechanism of graphyne-intracellular protein interaction for regulation of the phenotype of macrophages. Relevant research results entitled "The Underlying Function and Structural Organization of the Intracellular Protein Corona on Graphdiyne Oxide Nanosheet for Local Immunomodulation" have been published in Nano Letters.
Tumor-associated macrophage (TAM) is the most numerous inflammatory cell group in a variety of tumors. It promotes tumor growth, metastasis and recurrence, as well as induces immunosuppression. Importantly, itis associated with poor prognosis of solid tumors. TAM depletion, macrophage recruitment blocking, and reprogramming are the main useful treantment methods that have been designed based on TAMs.
Nowadays, a variety of small molecule drugs are employed in clinical trials, but small molecules lack targeting ability. As we all know, nanomaterials/nano drugs will face a complex physiological environment after entering the body. They interact with the surrounding biological fluids or biological molecules (proteins, DNA and lipids).
Due to their excellent unique physical and chemical properties, many novel nanomaterials are designed as delivery vehicles and immunomodulators to improve the tumor immunosuppressive microenvironment. Graphdiyne oxide (GDYO) as an emerging 2D carbon-network nanomaterial have offered a wide range of applications in catalysis, energy, biomedicine and other fields. GDYO comprises of hybridized sp and sp2 carbon atom and the surface consists of well-arranged C=O and C-OH groups, making GDYO behave in an unpredictable manner at the nano-bio interface.
Herein, researchers use the isotope 13C to label GDYO and quantitatively analyzes the interaction ratio between GDYO and intracellular proteins. It is found out that a unique protein corona is formed on the surface of GDYO in macrophages, which is highly enriched in signal transduction and activator of transcription (STAT3) protein.
STAT3 is an important signal transduction protein and transcription factor in cells. It is closely related to the occurrence and development of tumors. By inhibiting its signal pathway, it can inhibit tumor growth and metastasis. By inhibiting the activation of STAT3 protein, GDYO reverses immunosuppressive M2 macrophages into pro-inflammatory M1 macrophages, improves the immunosuppression caused by TAMs, increase the infiltration and activation of killer T cells, and improve the efficacy of PD-L1 antibodies.
Additionally, the distribution and metabolism of GDYO in peritoneal macrophages and tumors after administration are analyzed.
The interaction at the GDYO–STAT3 interface, driven by structure matching, hydrogen bonding and salt bridges, simultaneously triggers the immune response in the tumor microenvironment. The distance between two adjacent C=O and/or C-OH groups is 5.5 Angstrom. The pitch of α-helix in the N-terminal domain of STAT3 protein is 5.4 Angstrom, which matches the distance of oxygen-containing groups. Furthermore, C=O and C-OH groups form hydrogen bonds or salt bridges with amino acid residues in the STAT3 protein.
In conclusion, researchers explain the molecular mechanism of the unique strong interaction of GDYO-STAT3. They comprehensively use proteomics, theoretical calculations, and isotope quantification technology to reveal for the first time the interaction mechanism of nanoparticle-protein interface in macrophages, which is useful for in-depth understanding of nano-biological interface regulation of complex biology.
Prof. CHEN’s group has long been engaged in the research of nanomaterials-biological interface. In 2011, they discovered that carbon tubes can rapidly adsorb proteins in the blood to form a corona to reduce biological toxicity (Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 16968-16973). In 2019, it was discovered that Gd@C82(OH)22 nanoparticles specifically bind to the complement component C1q protein in the blood of lung cancer patients, and activate the innate immune response by changing the structure of the C1q molecule (Nano Lett. 2019, 19, 7, 4692-4701). In 2021, the unique in vivo transportation, metabolism and bioavailability of molybdenum disulfide nanomaterials mediated by protein corona was reported for the first time (Nat. Nanotechnol. 2021, 16, 708-716). These studies provide key and cutting-edge analytical methods for nano-biological effects and nano-medicine research, and vigorously promote the development of nano-biomedicine.
This work was financially supported by the National Key Research and Development Program, the National Natural Science Foundation of China, the Strategic Priority Research B Program of the Chinese Academy of Sciences, and the research and development projects in key areas of Guangdong Province.
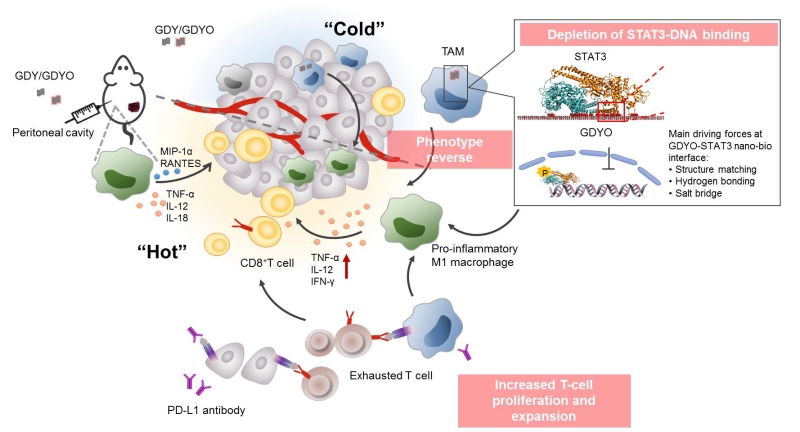
Figure. Graphyne oxide improves tumor immunosuppressive microenvironment through interaction with its intracellular protein corona
Contact
CHEN Chunying
National Center for Nanoscience and Technology
E-mail: chenchy@nanoctr.cn




