Cancer-associated thrombotic complications are the second leading cause of death in cancer patients. Nevertheless, the precise mechanisms underlying the development of cancer-associated thrombosis remains elusive owing to the lack of specific tools or methods to reliably examine the variation of highly associated clotting markers.
The interplay between platelets and tumor cells was shown to result in hyperactivity or activation of circulating platelets in cancer patients, ultimately resulting in formation of cancer-associated thrombus. The incidence of cancer-associated thromboembolic events is heavily dependent on the adhesion ability of platelets to fibrin network, which is determined by the degree of platelet activation. However, a major limitation in platelet activity state testing is the difficulty to replicate biorheological conditions in vitro, which incorporate the effects of hemodynamic forces such as pressure, flow, and shear stress.
To address this challenge, in a recent study published in Cell Reports Methods, the research group led by Prof. NIE Guangjun and Prof. LI Suping from National Center for Nanoscience and Technology (NCNST) developed a fibrin-network-containing microfluidic device (which models the dynamic flow in the living vessels) that can simultaneously measure the affinity of millions of individually circulating platelets for fibrin architecture. Using this device, the researchers precisely characterized platelet activity status over tumor progression, thus realizing cancer-associated thrombus prediction so as to guide timely anticoagulation treatment to reduce cancer mortality.
The researchers fabricated devices containing four parallel channels and infused a cold mixture of thrombin and fibrinogen into the devices to generate a uniform fibrin network. After optimizing the fibrin network porosity and flow rate, the microfluidic device selectively captured the tumor-associated hyperactive/activated platelets while allowing resting platelets to pass through the channels, and assessed the binding ability of platelets for fibrins during flow-through under shear stress.
Using multiple preclinical animal tumor models and clinical patient samples, the microfluidic devices showed a higher performance in distinguishing tumor-associated platelet activity than the conventional coagulation tests, and demonstrated with high sensitivity that platelet activity increased with tumor progression. In particular, using patient platelet samples, the researchers further validated that platelet hyperactivity measured by microfluidic device could predict the incidence of cancer-associated thrombosis with high sensitivity and specificity.
Furthermore, the microfluidic device also exhibits a potential to more precisely discriminate aberrant coagulation status in stroke and pulmonary embolism mouse models. The device can be further developed for predicting virus infection-associated thrombosis incidence, thus directing antiplatelet or anticoagulation therapy in multiple coagulation disorders.
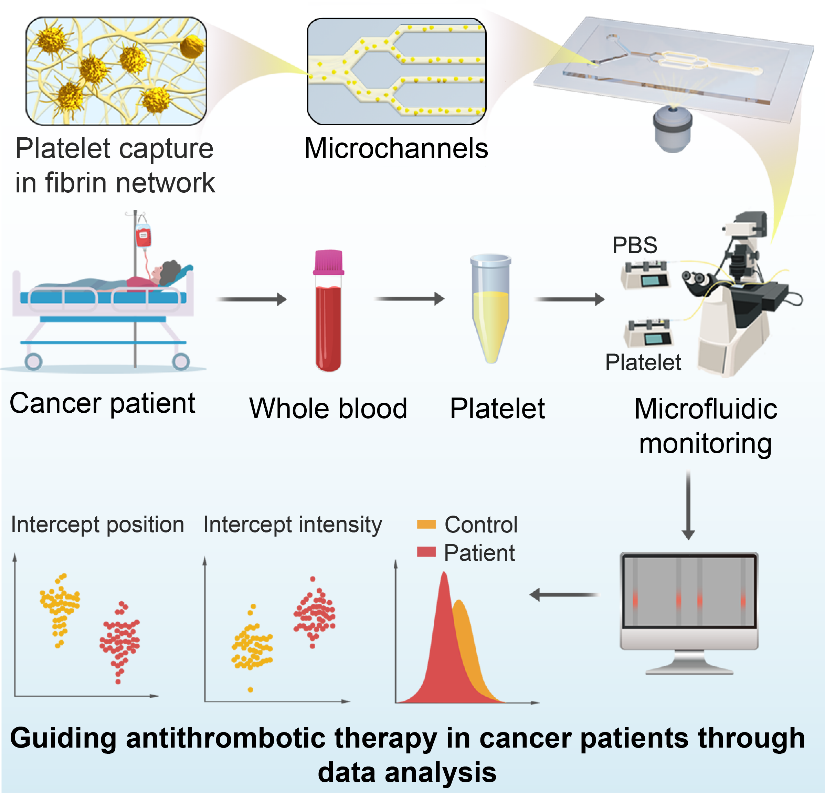
Schematic diagram of the sample collection and working principle of the microfluidic device (Image by the research group)
Contact:
Guangjun Nie and Suping LI
National Center for Nanoscience and Technology (NCNST)
E-mail: niegj@nanoctr.cn; lisuping@nanoctr.cn




