mRNA-based vaccine has emerged as a promising alternative for tumor immunotherapy in recent years, owing to its advantages in safety, personalized design, and industrial production. Efficient cytosolic delivery and translation of mRNA antigens for cross-presentation by antigen presenting cells (APCs), along with robust stimulation of the immune system, are essential for orchestrating effective tumor-specific immunity. Scientists have consistently focused on utilizing delivery systems—such as ionizable lipid nanoparticles (LNPs)—to increase cellular uptake and facilitate endo-lysosomal escape to maximize the availability of mRNA vaccine. However, it remains that only 1 to 2% of RNAs were released from endosomes. Moreover, the half-life of free mRNA released from these delivery systems is typically transient, which limits the yields of antigen protein expression. Few studies have addressed the critical issue of enhancing translation efficiency of mRNA within cytoplasm, a significant yet often overlooked aspect in mRNA delivery. Therefore, improving the translation efficiency of the limited cellular mRNA copies is essential for a higher protein expression and the subsequent immune efficacy of cancer mRNA vaccine.
CHEN Chunying’s group at the National Center for Nanoscience and Technology (NCNST) has dedicated to the development of nanocarriers for nucleic acid delivery, aiming to apply for immunotherapy etc. On Dec. 22, a paper authored by CHEN Chunying et al., titled "mRNA Compartmentalization via Muti-Module DNA Nanostructure Assembly Augments the lmmunogenicity and Efficacy of Cancer mRNA Vaccine", was published in Science Advances. This study developed a mRNA compartmentalization via multi-module DNA nanostructure (MMDNS) assembly, which not only promoted the mRNA expression efficiency but also enhanced adjuvant efficiency thereby augmenting both the immunogenicity and efficiency of mRNA vaccines.
The research team constructed the MMDNS-based mRNA vaccine utilizing a programmable DNA hybridization chain reaction (HCR) strategy, with integrating antigen-coded mRNA, CpG oligodeoxynucleotides (ODNs), an acidic-responsive DNA sequence, and dendritic cells (DCs) targeting aptamer. Following intramuscular injection into mice, the MMDNS-based mRNA vaccines were efficiently internalized by DCs mediated by targeting aptamers. MMDNS undergoes in situ assembly within acidic lysosomes to form micro-sized aggregates, inducing an enhanced CpG ODN adjuvant efficacy. Subsequently, the aggregates escape into the cytoplasm, providing a moderate compartment that supports the efficient translation of spatially proximal mRNA transcripts by localizing relevant reaction molecules. The mRNA compartmentalization-based vaccine boosts a strong immune response and effectively inhibits tumor growth and metastasis, offering a robust strategy for cancer immunotherapy.
“This study innovatively utilizes dynamic assembly of multi-module DNA nanostructure to construct mRNA compartment within cells, optimize mRNA expression microenvironment, enhance antigen expression and specific cross-presentation, providing an alternative strategy for developing efficient mRNA nanovaccines and improving the immune efficacy of tumor vaccines.” states CHEN Chunying.
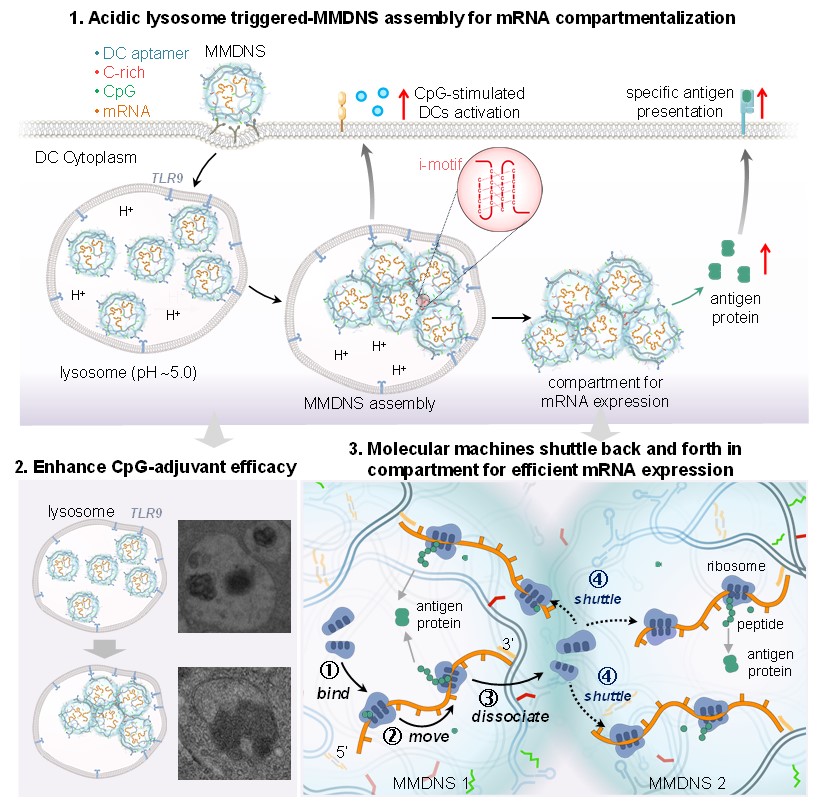
Fig.1 Construction of mRNA compartmentalization–based cancer vaccine which promoted the mRNA expression efficiency as well as enhanced adjuvant efficiency thus augmenting immunogenicity and efficiency of mRNA vaccines. (Image by CHEN Chunying et al)
Contact:
CHEN Chunying
National Center for Nanoscience and Technology (NCNST)
E-mail: chenchy@nanoctr.cn




